Stories
Posted date
2020.11.20PharmaEssentia Corporation

Developed by PharmaEssentia Corporation (6446), Besremi (P1101), a novel, long-acting, mono-pegylated proline interferon, markets well globally, winning the Go-Global Award - Gold Medal Award in the 2020 Taipei Biotech Awards. The European Medicines Agency (EMA) granted marketing authorization for Besremi in February 2019. It was the first EMA-approved new biologics in Taiwan. This May and July, PharmaEssentia Corporation (PharmaEssentia) also obtained Taiwanese and Swiss licenses to sell Besremi.
Founded in October 2003 by a group of Taiwanese-American executives and high-ranking scientists, PharmaEssentia develops treatments for myeloproliferative neoplasms, hepatitis, and other serious cancers using a novel pegylation technology platform and small-molecule drugs through transnational cooperation. Based in Taiwan, PharmaEssentia has its lab and factory conform to the EMA GMP, making it the glory of Taiwan’s biotechnology industry.
Besremi is used to treat the polycythemia vera (PV) without splenomegaly. It is the world's first interferon therapy approved for PV. PharmaEssentia has authorized partners to sell Besremi in Europe, with the sales revenue reaching NT$305 million last year.
Besides the COVID-19 pandemic, the interim report on clinical trials for patients with low risk of PV in the Annual Congress of European Hematology Association verifies that the use of Besremi to treat patients with low risk of PV has a tremendous effect compared with the bloodletting therapy, which will increase the demand for P1101 in Europe. The safety and efficacy of P1101 have been widely recognized by physicians and patients. PharmaEssentia expects to obtain marketing authorization for Besremi in other European countries.
PharmaEssentia’s global presence: subsidiaries in the United States, Japan, South Korea, and China and partners in Europe (source: organizer)
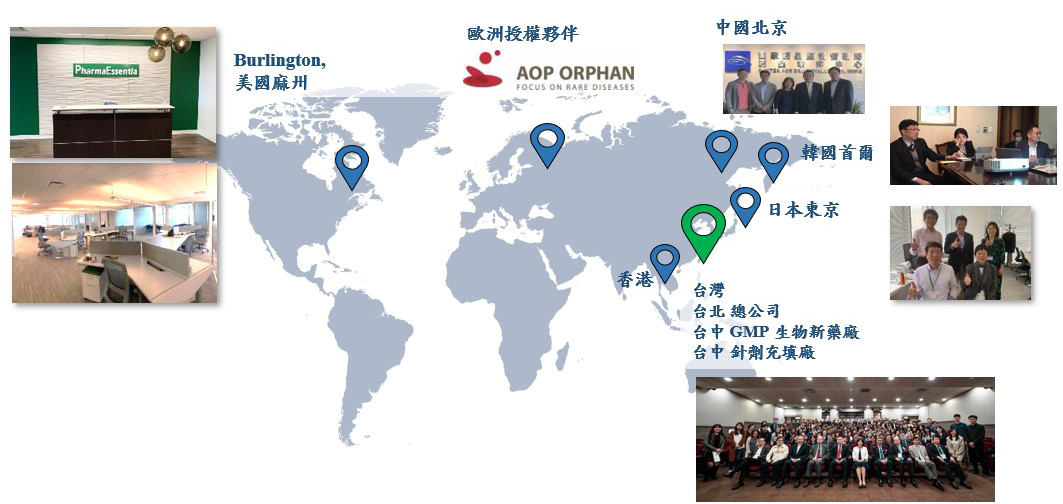
On May 13, 2020, the U.S. FDA accepted the biologics license application (BLA) for Besremi for treatment for PV submitted by PharmaEssentia on March 13. Granting the BLA is expected on March 13, 2021. PharmaEssentia has obtained licenses in five states of America, including Massachusetts, Maryland, Oregon, North Dakota, and Washington, and will continue to apply to other states for licenses. It supply chain is primed to ensure that Besremi is available for sale after the BLA is granted in March next year. Different from the licensing strategy adopted in Europe, PharmaEssentia has a team dedicated to selling Besremi in the United States. The results are yet to be examined.
At the end of July 2020, the Ministry of Food and Drug Safety of South Korea qualified the subsidiary for the orphan drug. For Besremi, an application for a drug license does not require a separate bridging study in South Korea, which saves time and costs for clinical trials and speeds up the application review. Advantages include conditional approval of drug licenses and priority review.
In May 2020, Besremi was granted a drug license in Taiwan. PharmaEssentia immediately applied to the National Health Insurance Administration for coverage of National Health Insurance (NHI). Although the application has not been granted, some patients have already benefited at their own cost.
With research and development, clinical research, manufacturing, and commercialization all based in Taiwan, PharmaEssentia is an one-stop pharmaceutical company that sells drugs globally. Subsidiaries in the U.S., China, Japan, and South Korea are also in place to recruit high-ranking and scientists executives specializing in myeloproliferative neoplasms (MPN). The goal is to sell MIT drugs globally.