Stories
Posted date
2020.11.20BRIM Biotechnology

BRIM Biotechnology, Inc.’s Innovative PEDF-derived Short Peptide (PDSP) Technology Platform launches to develop the application of multiple indications. BRM421, eye drops for dry eyes, is used as the first indication for clinical efficacy verification. The phase 2 clinical trials in the United States have been completed. In the 3C era, the need of treatment for eye diseases is increasing rapidly, which will contribute to the development of new drugs. BRIM Biotechnology, Inc. (BRIM) wins the Innovation Award - Gold Medal Award in the 2020 Taipei Biotech Awards for this innovative platform.
BRIM is a virtually operating company established in 2013 in Taiwan, focusing on developing new drugs in various areas through translational science. Over the past seven years, BRIM has extensively sought drug candidates from academic and research institutions at home and abroad. After rigorous selection and development, BRIM’s first Innovative PEDF-derived Short Peptide (PDSP) Technology Platform is internationally patented and applicable to multiple indications with complete international patents.
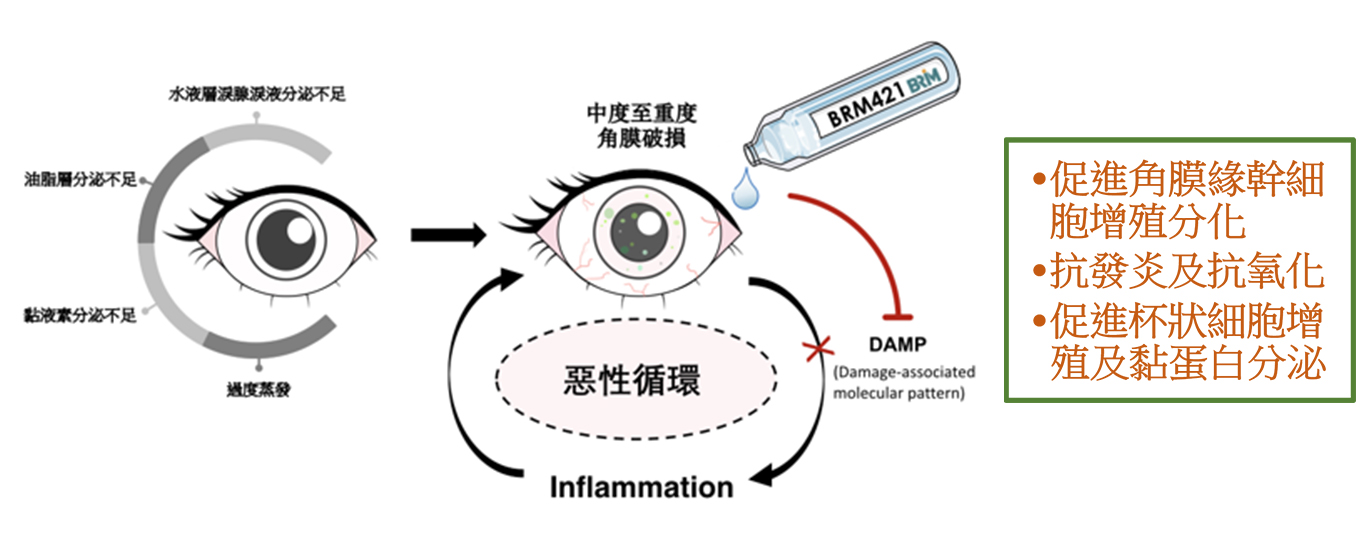
The Innovative PEDF-derived Short Peptide (PDSP) Technology Platform has some unique features that make it well suited for the discovery and development of therapeutic new drug application, including (1) promoting the proliferation and differentiation of stem cells and then repairing damaged tissues; (2) low immunogenicity due to short peptides; (3) showing early-onset potential in various disease animal models; and (4) providing no endotoxin risk and high pharmaceutical stability with drug substance produced by solid-phase peptide synthesis and formulation optimization. These special properties of the platform can be used to develop the application of multiple indications.
BRM421 for treatment for the dry eye syndrome has the following functions: (1) promoting the proliferation of limbal stem cells and then repairing damaged tissues; (2) promoting the proliferation of goblet cells to restore mucin secretion and tear quality; and (3) anti-inflammatory and antioxidant. With systemic exposure, BRM421 is granted for phase 2 clinical trials on first-in-human (FIH) in February 2017. In June 2017, 157 patients completed clinical safety and efficacy trials. The test results prove that BRM421 is highly safe and can significantly improve the quality of the tear film, burns, and vision of patients. The subgroup analysis, which compiles the statistics on the sign and symptom specific to moderate to severe patients, indicates an early-onset effect and improvement in dryness and vision.
BRM421 is currently on trial by 200 patients (clinical phase 2/3) in the United States; in addition, BRIM has completed authorization in China, Hong Kong, and Macau and introduced strategic investors. Generous licensing revenue is expected to contribute to the Innovative PEDF-derived Short Peptide (PDSP) Technology Platform for the subsequent development of new drugs for other new indications, including BRM423 (severe corneal injury) and BRM521 (osteoarthritis, OA). BRIM also evaluates potential new drugs at home and abroad and extends to the clinical stage for authorization upon proof of concept. It actively participates in international business trade fairs to seek opportunities for authorization or cooperation through local partners.
In the future, the Innovative PEDF-derived Short Peptide (PDSP) Technology Platform is poised to develop drugs for external use and injections for muscle regeneration, corneal wound healing, OA, etc. based on different indications and patient needs.
Innovative PEDF-derived Short Peptide (PDSP) Technology Platform
BRIM has entered into several contracts for the development of highly innovative drug platforms with partners and has established a startup, Ascendo Biotechnology, Inc., for two of them.
In the future, BRIM will continue developing new drugs while strengthening risk management to improve the efficiency and success rate of drug development. To fulfill its corporate social responsibility, BRIM strives to cultivate cross-field professionals in drug development and forward clinical trials based on the research results. Despite long development time, high risk, and a considerable sum of money, BRIM is adamant that it will succeed in developing new drugs.