Roadshow
BRM421
Dry Eye Disease (DED) is a common disorder in which the eyes produce insufficient tears to lubricate the eye. It can stem from pre-existing eye abnormalities, previous disease, or medical intervention. Aside from dry eyes, the most common symptoms of moderate-to-severe DED are eye fatigue, headaches, neck/shoulder pain, and blurred vision. In some cases, patients experience other symptoms including redness, burning sensations, or constant pressure on the eye. DED is increasing globally with an estimated 1.52 billion cases worldwide in 2020*. There is currently no cure for Dry Eye Disease and there is a lack of effective therapies available to patients.
Speeds up corneal repair
BRM421 is a developing topical ophthalmic solution for patients with moderate-to-severe DED. The active pharmaceutical ingredient of BRM421 is a synthetic peptide comprised of 29 amino acids derived from Pigment Epithelium-Derived Factor (PEDF) with neurotrophic and anti-inflammation properties. PEDF is a secreted glycoprotein with numerous known biological effects, including the promotion of tissue regeneration at the ocular surface. BRIM’s PEDF-derived Short Peptide (PDSP) has been shown to promote the growth and expansion of limbal epithelial stem cells in severe ocular wounds in both mice and rabbits. BRM421 has a unique mechanism of action. It stimulates the proliferation and differentiation of corneal limbal stem cells, and as a result, speeds up the corneal repair process. It also promotes the proliferation and differentiation of goblet cells, improving the tear quality of DED patients.
BRM421 entered Phase 3 trials in 2022
BRIM has completed two Dry Eye Disease clinical studies, a First-in-Human (FIH) Phase 2 study and a Second-in-Human (SIH) Phase 2/3 study. These two studies have shown BRM421 to be a safe and, effective Dry Eye Disease therapy, with early on-set action. BRM421 entered a Phase 3 study at the end of 2022 and is expected to file an NDA in 2026.
* Statistics from Datamonitor Healthcare

- Established 2013
- Company BRIM Biotechnology, Inc.
- Telephone +886-2-2659-8586
- Address 8F, No.1, Alley 30, Lane 358, Ruiguang Rd.,Neihu Dist., Taipei 11492, Taiwan
BRIM translates research into clinical assets with the highest potential to positively impact patient lives. BRIM primarily targets highly novel projects with new molecular entities and niche platforms, targeting diseases where there are unmet medical needs. This involves, in-licensing from, or partnering with, academic institutions and biotech or pharma companies, both locally and internationally.
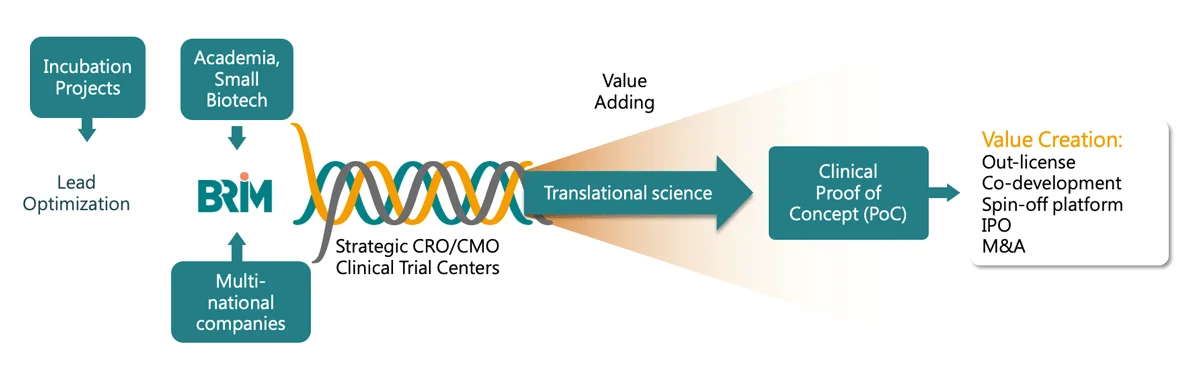
De-risking is the essence of value creation, delivered through our scientific expertise and our virtual team model. An internal integrated team of project management professionals and translational science experts provide expert input and operational leadership while outsourcing research activities to external entities such as research institutes, CROs, and CMOs.
BRIM’s model of connectivity, shared knowledge and scientific excellence means development times and costs are dramatically reduced. Upon reaching the clinical proof of concept (PoC), we out-license or partner with the best-suited third party to continue development through market approvals and commercialization, maximizing profit through royalties, milestones, or profit sharing. Our efficient business model delivers maximum ROI and is not limited by the traditional pharma R&D approach.
All drug development activities are executed according to global regulatory requirements and standards. Clinical trials are conducted to meet requirements for regulatory submissions to the US FDA through the help of CROs, CMOs, CDMOs, and partnership networks (e.g., hospitals, KOLs, government-sponsored agencies, research institutes,…etc.). BRIM’s translational science expertise enables rapid drug development and regulatory progression while ensuring high-quality standards.
